Learning from the team-NB survey 2021
The Team-NB Survey 2021
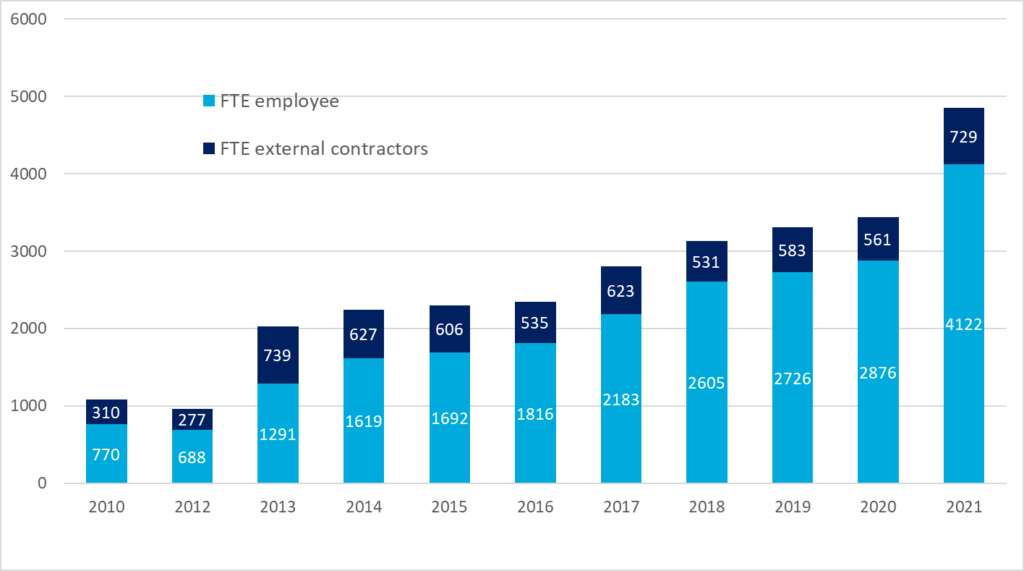
What do Notified Bodies do to face this situation?
Notified Bodies have increased their personnel, as well as added external contractors to their teams. They are looking for more personnel, but it is difficult due to the stiff competition with manufacturers and consulting companies.
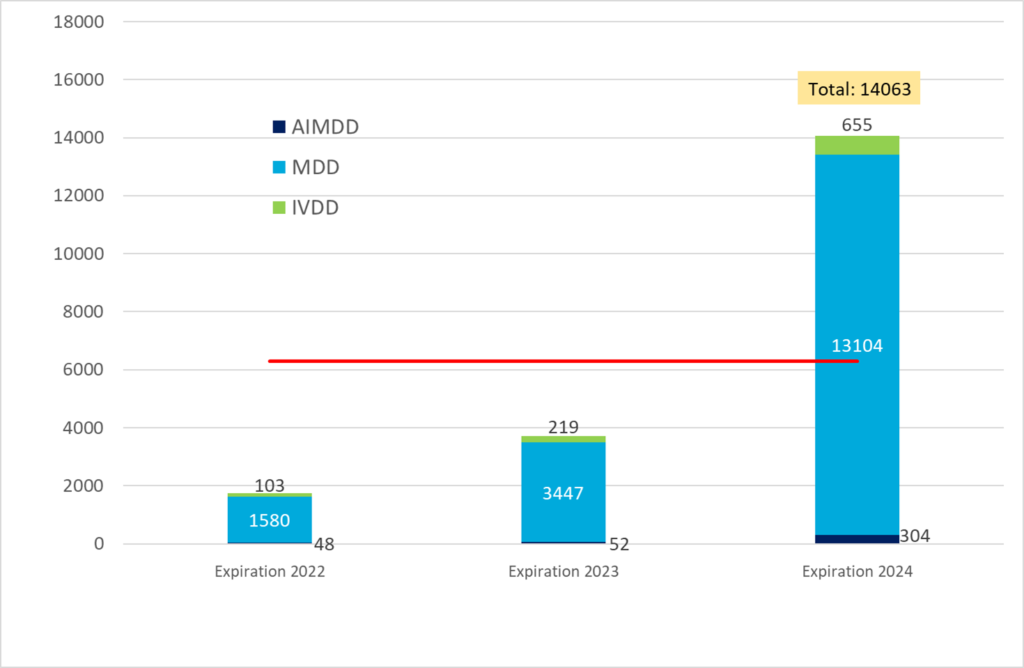
Notified Bodies are encouraging all manufacturers to make applications now so that they are not disappointed when their Directive certificates expire.
What can manufacturers do?
Applications for MDR certificates are low considering that there are only two years remaining until May 26, 2024. If you hold an MDD certificate remember that you still have to go through numerous QMS and TD review rounds. Otherwise you risk that you product cannot be sold continuously. Make your application now. And be aware that this also applies to in-vitro diagnostics. Maybe even more so, because fewer NBs are designated for IVDR.
75 % of notified bodies indicate that at least half of the TD submitted are deemed incomplete. This is especially troublesome since in those cases NBs can’t even start with the assessment but have to request additional information instead. So, prepare well for your submission. Make sure you submit all documents.
Use smart tools and work with experts. Sometimes you run the risk of not seeing the wood for the trees. Then it can be worth its weight in gold to have an external expert look at your technical documentation and identify the gaps that still need to be filled. They can identify key documents and help you to focus your resources to get you through the NB review as smooth as possible. For example to focus on the heart of your technical documentation: the clinical evaluation, risk management, labelling and GSPR.
Yes, external experts have their price, but they are not as expensive as expedited NB reviews. And if they are competent, then they get your documents ready faster than you could on your own. This will safe time. Additionally, if your documents are already (near-) perfect before you submit them to your notified body, you will have much fewer review rounds. This will again safe time and lots of money.
(1) PRESS RELEASE Team-NB sector survey 2021 May 11th 2022 (edited):
Source: https://www.team-nb.org/wp-content/uploads/2022/05/Team-NB-MD-Sector-Survey-PressRelease-20220516.pdf
(2) Team-NB Medical Device Survey 2021 – Data from all members (end 2021)
Source: https://www.team-nb.org/wp-content/uploads/2022/05/Survey-2021-20220516-1.pdf
